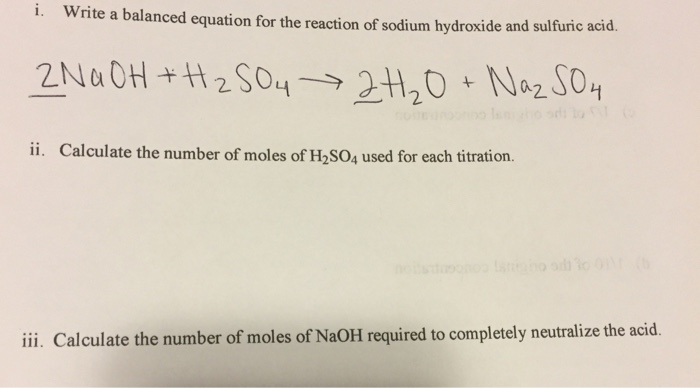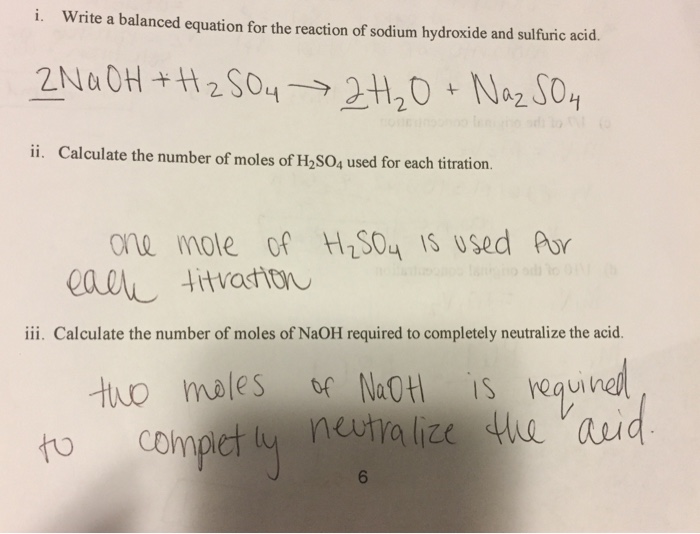The mass of NaOH required for complete neutralisation of 1 g molecules of H2SO4 is ? (molar mass of NaOH = 40 g/mole, H2SO4 = 98 g/mole) Given reaction 2NaOH + H2SO4 → Na2SO4 + 2H2O
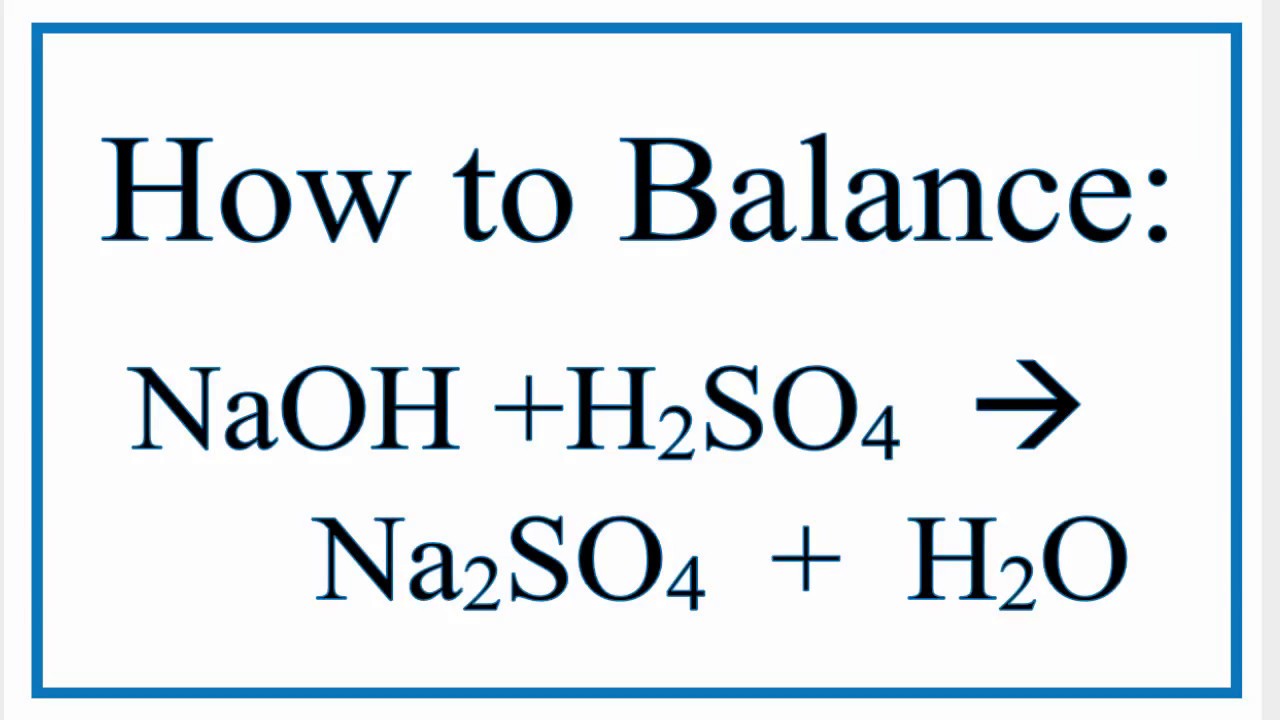
Balance the following chemical equations: i. NaOH + H2SO4 → Na2SO4 + H2O - Sarthaks eConnect | Largest Online Education Community
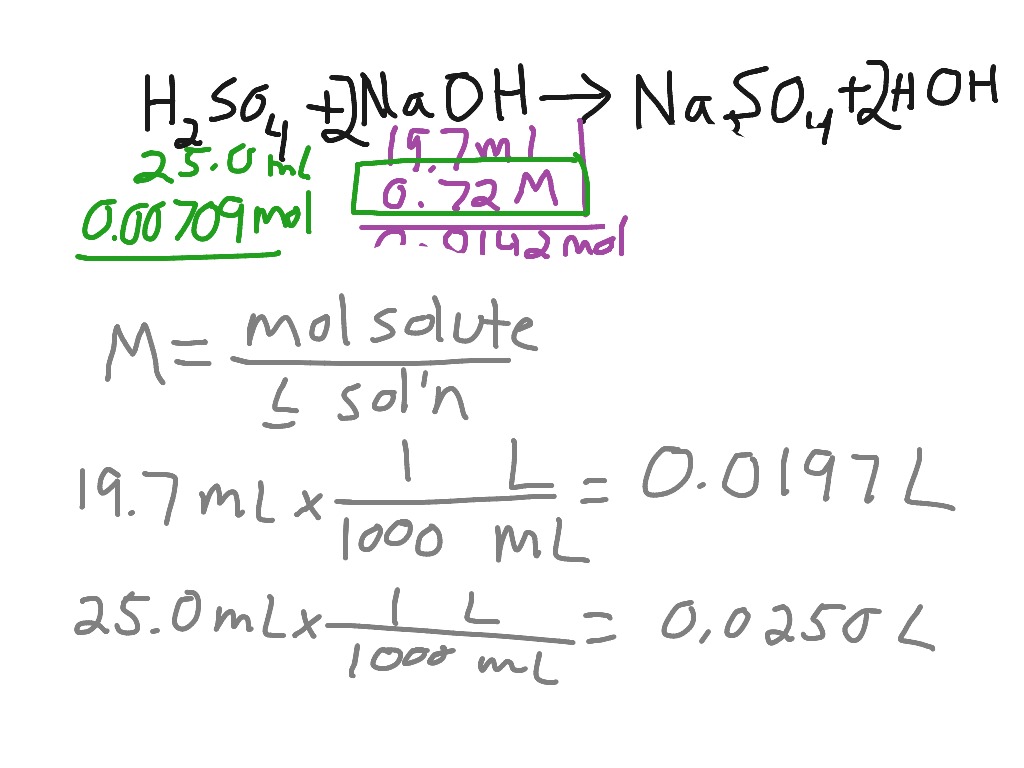
Titration of sulfuric acid with sodium hydroxide | Chemistry, Acids and Bases, Stoichiometry | ShowMe

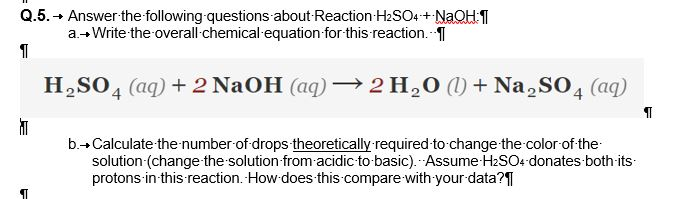
Question Video: Calculating the Volume of Sulfuric Acid That Completely Neutralizes a Given Volume and Concentration of Sodium Hydroxide | Nagwa

NaOH + H2SO4 → Na2SO4 + 2H2O 2 mol 1 mol 1 mol 0.5 mol = 49g H2SO4 (pure) Thus, 70% H2SO4 required = 49 x 70 = 70g 70